Styrene (shown below) has 8 π electrons but is aromatic. Why? 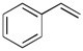
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q51: Rank the following in order of increasing
Q52: Use resonance structures to explain why the
Q53: Classify pyrrole as aromatic, antiaromatic, or nonaromatic.
Q54: Is the molecule below aromatic, antiaromatic, or
Q55: Which sequence correctly ranks the following substrates
Q57: Classify the compound below as aromatic, antiaromatic,
Q58: Why would the reaction below proceed at
Q59: 3-Chlorocyclopropene is solvolyzed in methanol at a
Q60: Which sequence ranks the indicated protons in
Q61: Classify the compound below as aromatic, antiaromatic,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents