Is the molecule below aromatic, antiaromatic, or nonaromatic? 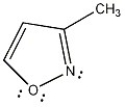
Correct Answer:
Verified
Q49: Classify the compound below as aromatic, antiaromatic,
Q50: Nitrogen's lone pair electrons occupy what type
Q51: Rank the following in order of increasing
Q52: Use resonance structures to explain why the
Q53: Classify pyrrole as aromatic, antiaromatic, or nonaromatic.
Q55: Which sequence correctly ranks the following substrates
Q56: Styrene (shown below) has 8 π electrons
Q57: Classify the compound below as aromatic, antiaromatic,
Q58: Why would the reaction below proceed at
Q59: 3-Chlorocyclopropene is solvolyzed in methanol at a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents