The formal charge on the oxygens in the compound below are ________. 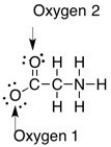
A) Oxygen 1: 0, Oxygen 2: 0
B) Oxygen 1: -1, Oxygen 2: 0
C) Oxygen 1: 0, Oxygen 2: -1
D) Oxygen 1: +1, Oxygen 2: 0
E) Oxygen 1: -1, Oxygen 2: -1
Correct Answer:
Verified
Q17: Orbitals which are equal in energy are
Q18: When filling two or more orbitals of
Q19: Draw a correct Lewis structure for chloromethane,
Q20: The element with the electronic configuration 1s22s22p63s1
Q21: Add the appropriate formal charge to each
Q23: Add the appropriate formal charge to each
Q24: One or more of the atoms in
Q25: The formal charge on oxygen in dimethyl
Q26: Draw a correct Lewis structure for acetonitrile,
Q27: Draw 2 possible Lewis structures for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents