Using a Frost circle,draw the molecular orbital energy diagram for the tropylium cation 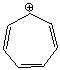 and predict if it is aromatic.
and predict if it is aromatic.
Correct Answer:
Verified
Q24: Which one of the following compound(s)is antiaromatic?
Q26: Classify the following compound as aromatic,antiaromatic,or nonaromatic.Explain
Q27: Which one of the following compounds is
Q28: Which one of the following statement
Q28: The number of π electrons present in
Q30: Classify the following compound as aromatic,antiaromatic,or nonaromatic.Explain
Q30: The number of π electrons present in
Q31: Which one of the following compound is
Q32: Classify the following compound as aromatic,antiaromatic,or nonaromatic.Explain
Q33: Which one of the following compounds is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents