The free radical polymerization of styrene with benzoyl peroxide yields a polymer that has repeat units arranged primarily in a 'head-to-tail' arrangement.This means that the phenyl group primarily ends up placed at alternating carbon atoms along the chain.Use correct arrow formalism to show why this arrangement is preferred over a 'head-to-head' or 'tail-to-tail' arrangement. 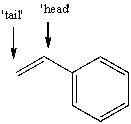
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q20: Use correct arrow formalism to show the
Q21: Compound A (C6H12)reacts with HBr/ROOR to give
Q23: Predict the major product(s)of the following reaction.
Q24: Which term best describes the process shown
Q26: Which of the following correctly describes the
Q26: Which intermediate leads to the major product
Q27: Upon treatment with NBS and irradiation with
Q28: Predict the major product(s)of the reaction shown
Q30: Compound A has molecular formula C9H20.Compound A
Q45: Use correct arrow formalism to show the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents