Based on the following energy diagram,is the following reaction exothermic or endothermic? 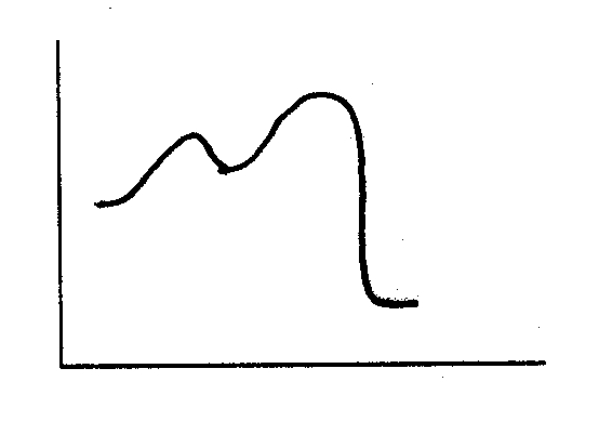
Correct Answer:
Verified
Q19: Predict the sign of
Q20: Why is entropy negative for ring closures?
A)Closing
Q21: Given the following rate law,the reaction is
Q22: Identify the nucleophilic atom in the following
Q23: Identify the nucleophilic atom in the following
Q26: What is the energy of activation for
Q28: What is the effect of a catalyst
Q29: Given the following rate law,the reaction is
Q36: Does a reaction with a
Q52: Draw an energy diagram for an endothermic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents