Multiple Choice
Could the amide anion (- NH2) deprotonate the following compound? 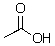
A) Yes
B) No
Correct Answer:
Verified
Related Questions
Q50: Can this base exist in water?
Q51: Can this base exist in hexane?
Q52: Can this base exist in water?
Q53: Could water protonate the following compound?
Q54: Could hydroxide deprotonate CH4?
A)Yes
B)No
Q56: What is the counterion of iodide in
Q57: Can this base exist in liquid ammonia?
Q58: Explain the leveling effect.
Q59: Could ammonia (NH3)deprotonate the following compound?
Q60: Could hydroxide deprotonate the following compound?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents