SN2 substitution at secondary halides and sulfonates is often complicated by competing E2 elimination.Which of the nucleophiles below would you choose to obtain the highest yield in an SN2 reaction with menthyl bromide? 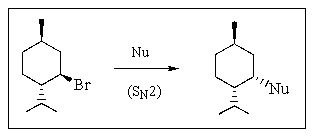
A) CH3ONa
B) CH3CO2Na
C) (CH3) 3N
D) (CH3) 3COK
E) C6H5SNa
Correct Answer:
Verified
Q21: Arrange the following in order of increasing
Q22: Predict the major product of the following
Q23: What is the best way to prepare
Q24: Predict the major product of the following
Q25: Predict the major product of the following
Q27: If the reaction rate of the following
Q28: What is the correct stereochemistry of the
Q29: Name the following compound: Q30: The curved arrows below represent what type Q31: Which of the following can be used![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents