Carbon dioxide is an essential greenhouse gas that insulates the planet and maintains a surface temperature that allows life to flourish.Human activities have resulted in a dramatic increase in the greenhouse gases in the atmosphere.Which of the following reactions produce the most carbon dioxide per mole of carbon-containing starting material? (Note that these equations are not balanced.)
A) 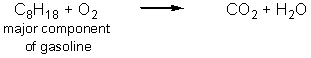
B) 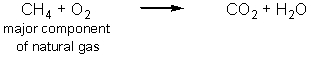
C) 
D) All produce the same amount of carbon dioxide.
E) None of these reactions produce carbon dioxide.
Correct Answer:
Verified
Q16: Given the relative reactivities of various kinds
Q17: What reactant is needed to complete and
Q18: In a carbon radical,the single unpaired electron
Q19: Which one of the reactions below will
Q20: Which of the following is not true
Q22: Which of the following would you not
Q23: Rank the following radicals in decreasing order
Q24: How many constitutional isomers can be formed
Q25: Which of the following hydrocarbons would
Q26: What reactant is needed to complete and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents