Chemists Have Determined That Chlorofluorocarbons Are Contributing to Destruction of the Protective
Chemists have determined that chlorofluorocarbons are contributing to destruction of the protective ozone layer.Upon irradiation of chlorofluorcarbons,chlorine radicals are produced and react with the ozone via the chemical process shown below.Which of the following statement(s) are true based upon these equations? 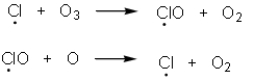
A) Termination steps in the equations above are contributing to ozone depletion.
B) A single chlorine radical is capable of destroying many molecules of ozone since it is regenerated.
C) Chlorine radicals are the result of a heterolytic cleavage of the C-Cl bond of the chlorofluorocarbon.
D) A,B and C are correct.
E) B and C are correct.
Correct Answer:
Verified
Q22: Which of the following would you not
Q23: Rank the following radicals in decreasing order
Q24: How many constitutional isomers can be formed
Q25: Which of the following hydrocarbons would
Q26: What reactant is needed to complete and
Q28: Which is most true about the function
Q29: Which alkane below would you expect to
Q30: Given the relative reactivities of various kinds
Q31: How many different products will result if
Q32: Give the relative reactivity in decreasing order
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents