The following reaction represents what type of process? 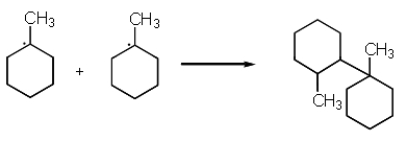
A) Propagation
B) Termination
C) Initiation
D) Nucleophilic addition
E) None of the above.
Correct Answer:
Verified
Q24: How many constitutional isomers can be formed
Q25: Which of the following hydrocarbons would
Q26: What reactant is needed to complete and
Q27: Chemists have determined that chlorofluorocarbons are contributing
Q28: Which is most true about the function
Q29: Which alkane below would you expect to
Q30: Given the relative reactivities of various kinds
Q31: How many different products will result if
Q32: Give the relative reactivity in decreasing order
Q34: In a free radical termination step:
A) an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents