What problem is encountered in the synthesis of ethyl acetoacetate (shown below) if sodium methoxide is used rather than sodium ethoxide? 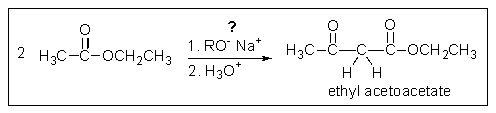
A) Sodium methoxide is too weak a base to cause this reaction to occur.
B) The condensation proceeds further to give tri-carbonyl products.
C) The reaction would produce some of both the methyl ester and the ethyl ester.
D) Sodium methoxide is too insoluble in methanol to promote the reaction.
E) Sodium methoxide is not basic enough to drive the reaction to completion.
Correct Answer:
Verified
Q1: What missing reactant would be required to
Q2: Which of the following would be the
Q4: Which of the following reaction sequences will
Q5: What product would be expected to result
Q6: What would be the expected product of
Q7: Predict the products of the following reaction.
Q8: Predict the major product of the following
Q9: What factors contribute to the stability
Q10: What would result from the reaction shown
Q11: Which of the following compounds will not
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents