Substitutions at sp2 carbons do not occur by an SN2 mechanism,but rather by way of a "tetrahedral intermediate." The tetrahedral intermediate shown could occur in which reaction? 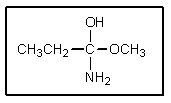
A) methyl propanoate + ammonia
B) propanamide + hydroxide
C) propanoic acid + methanol
D) propanamide + ethanol
E) None of these.
Correct Answer:
Verified
Q29: When compound X is heated with aqueous
Q30: What is the product of combining pentanoic
Q31: What would be the other product of
Q32: Which of the following reactions will produce
Q33: Which of the following is a lactam?
A)
Q35: What is the relative reactivity of carboxylic
Q36: What structure is not an intermediate in
Q37: Place the following compounds in order of
Q38: Which of the following reactions will not
Q39: What would be the products of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents