The addition of either the methyl Grignard reagent or methyllithium to camphor,followed by hydrolysis,produces a tertiary alcohol known as 2-methylisoborneol,an algal metabolite which imparts a musty odor to water at very low concentrations.However,the yield of alcohol does not exceed 50%,and large amounts of camphor are recovered from the reaction even when a large excess of the Grignard or lithium reagent are used.What would be the most plausible explanation? 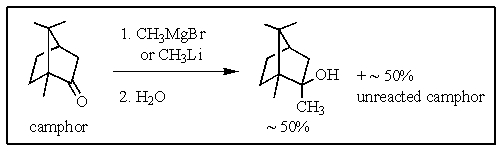
A) Ketones do not react with Grignard or lithium reagents.
B) The Grignard or lithium reagent had obviously degraded during storage.
C) The mechanism requires that,for each alcohol formed,one ketone molecule must remain unreacted.
D) With hindered ketones,the organometallic reagent could function as a base rather than as a nucleophile.
E) Alcohol formed early in the reaction could form an unreactive hemiacetal with remaining ketone.
Correct Answer:
Verified
Q9: Which product would you not expect in
Q10: Indicate to which side,if any,the following equilibrium
Q11: Predict the major organic product of the
Q12: Which of the following sets of reaction
Q13: Which set of reagents would be needed
Q15: To which side,if any,would the following equilibrium
Q16: Predict the major organic product of the
Q17: To which side,if any,would the following equilibrium
Q18: What would be the expected product of
Q19: What major product would you expect from
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents