A hydrocarbon with the molecular formula C7H12 exhibits the following spectroscopic data: 1H NMR = 1.3 (m,2H) ,1.7 (m,4 H) ,2.2 (m,4H) ,and 4.8 (quin,J = 3 Hz,2H) ppm; 13C NMR: = 26.8,28.7,35.7,106.9,and 149.7 ppm.The IR spectrum has key absorbances at 3072,2950,and 1649 cm-1.Hydrogenation of the compound furnishes a product with the molecular formula C7H14.Which structure is consistent with this data?
A) 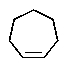
B) 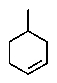
C) 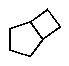
D) 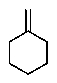
E) None of the above.
Correct Answer:
Verified
Q13: How many degrees of unsaturation are present
Q14: Predict the major product of the following
Q15: Which of the following are of lowest
Q16: Which of the following most accurately describes
Q17: Which of the following are (Z)isomers?
Q19: The following molecule contains how many units
Q20: Which of the following alkenes of the
Q21: Which structure would be named 3-bromo-5-methylcyclohexene?
A)
Q22: Which of the following are (E)isomers?
Q23: Predict the product of the following reaction:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents