Consider the following reaction. 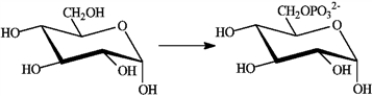 Which of the following describes this reaction?
Which of the following describes this reaction?
A) is so strongly exergonic that it does not require a catalyst
B) is an exergonic reaction not coupled to any other reaction
C) is an endergonic reaction that takes place because it is coupled to the exergonic hydrolysis of ATP
D) is an exergonic reaction that is coupled to the endergonic hydrolysis of ATP
Correct Answer:
Verified
Q1: Which enzyme catalyzes a reaction that is
Q2: In the course of the conversion of
Q2: The order of compounds in the conversion
Q3: Which group of small molecules best fit
Q5: Consider the following reversible reaction. 
Q6: In the reaction catalyzed by aldolase, the
Q7: Biotin is important in gluconeogenesis for all
Q11: Which of the following are not involved
Q19: During the complete catabolism of a molecule
Q29: Which enzyme is used in gluconeogenesis, but
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents