For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
-Which of the following compounds would produce the most downfield signal in a 13C NMR spectrum? A 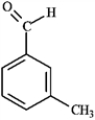 B
B 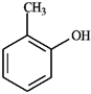 C
C 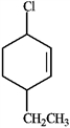 D
D 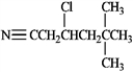
A) A
B) B
C) C
D) D
Correct Answer:
Verified
Q3: For each of the compounds below tell
Q4: Predict the splitting patterns you would expect
Q5: Which of the following would not produce
Q6: For each of the compounds below tell
Q9: Nuclear magnetic resonance spectroscopy provides information about
Q10: For each of the compounds below tell
Q11: For each of the compounds below tell
Q13: For each of the compounds below tell
Q56: A compound with the molecular formula C5H12O
Q60: Instructions: Refer to the structure of 3-methylbutan-2-one
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents