Use the first step of the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following question(s) . 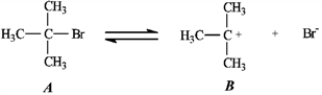
-In the following generic reaction between a halogen (X) and an alkane (R) , which of the following steps occurs slowly due to low concentration of reactants?
A) X⎯X → 2X∙
B) X∙ + R → HX + R∙
C) R∙ + X⎯X → RX + X∙
D) X∙ + R∙ → RX
E) X∙ + X∙ → X⎯X
F) R∙ + R∙ → R⎯R
Correct Answer:
Verified
Q1: Identify the functional groups present in each
Q2: Classify each structure below as a nucleophile
Q3: Polarizability:
A) explains a nonpolar carbon−sulfur bond participating
Q5: Which of the following correctly compares the
Q6: Add curved arrows to the following reaction(s)
Q7: Use the first step of the reaction
Q8: The reaction below is commonly used as
Q9: In theory, upon reaction with water in
Q10: Identify and label the nucleophile and electrophile
Q11: Use the first step of the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents