Add curved arrows to the following reaction(s) to indicate the flow of electrons in each.
-Consider the following reaction: 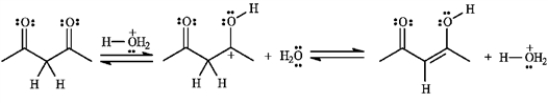 a)Assuming that the first step is the slow step, draw and label a qualitative energy diagram for the reaction.
a)Assuming that the first step is the slow step, draw and label a qualitative energy diagram for the reaction.
b)If the second step of the reaction were the slow step, briefly explain how the values of ΔG‡1, ΔG‡2, and ΔG° would change.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q5: Match each definition to one of the
Q13: Match each definition to one of the
Q29: Match each definition to one of the
Q30: Match each definition to one of the
Q31: Add curved arrows to the following reaction(s)
Q33: In the reaction below:
a)Label the nucleophile (Nu)
Q34: In the reaction below:
a)Label the nucleophile (Nu)
Q36: Use the reaction energy diagram below to
Q38: Use the second and third steps of
Q39: Use the reaction energy diagram below to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents