Consider the structure of acetic acid shown below. 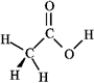 In the electrostatic potential map of acetic acid, in which of the following bonds would the terminal atom appear as the deepest shade of red?
In the electrostatic potential map of acetic acid, in which of the following bonds would the terminal atom appear as the deepest shade of red?
A) C=O
B) C−H
C) C−C
D) O−H
Correct Answer:
Verified
Q13: Label the acid and base in each
Q14: Indole is pleasant smelling in highly dilute
Q15: Refer to the following equation to answer
Q17: Phenylalanine is an amino acid that is
Q19: Circle the Lewis bases in the group
Q20: Explain why phenol has a lower pKa
Q21: Write an equation for the reaction of
Q22: The visual pigment in animal cells consists
Q23: Guanidine is a fairly strong amine base
Q36: Instructions: Refer to the following equation to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents