The condensed structure for dimethyl ether looks symmetrical. However, dimethyl ether has a dipole moment. Draw a structure that explains this and indicate the expected direction of the molecular dipole moment. 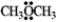 dimethyl ether
dimethyl ether
Correct Answer:
Verified
Q24: Which of the following statements is not
Q25: In aromatic nitration reactions, nitric acid (HNO3)
Q26: Draw the conjugate base of each species.
Q27: Identify the reactants and product in the
Q28: Write an equation for the reaction of
Q30: Use the curved arrow method to show
Q31: Rank the following in order of decreasing
Q32: Which is the strongest base (pKa values
Q33: Consider the molecules below to answer the
Q34: Complete this acid-base reaction, and label the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents