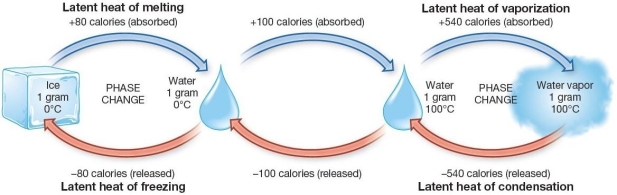
 Latent Energy absorbed and released during phase changes of water
Latent Energy absorbed and released during phase changes of water
Which of the following is true regarding the evaporation of water?
A) It requires the addition of 540 cal for each gram under normal sea level pressure.
B) It requires the loss of 540 cal for each gram under normal sea level pressure.
C) It requires the addition of 100 cal for each gram under normal sea level pressure.
D) It requires the loss of 100 cal for each gram under normal sea level pressure.
E) No latent energy is released or absorbed.
Correct Answer:
Verified
Q34: Q35: Assuming a Northern Hemisphere station,in which of Q36: Q37: Relative humidity is Q38: If the amount of water vapor in Q40: As temperature increases,the amount of energy available Q41: The dry adiabatic rate (DAR)is Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
A)the amount of water vapor
A) 6 C°

