The stability of 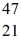 Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 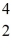
He: 4.002603 u 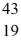
K: 42.960717 u 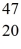
Ca: 46.954543 u 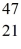
Sc 46.952409 u 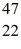
Ti: 46.951764 u
The 
Sc nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+ decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
Correct Answer:
Verified
Q42: A certain substance has a half-life of
Q43: The stability of Q44: An archaeologist finds the 14C in a Q45: The unstable isotope 234Th decays by β Q46: A hospital patient has been given some Q49: In a laboratory accident a work area Q49: How many days are required for a Q51: An isotope of Tc having a half-life Q57: The material used in certain nuclear bombs Q76: Today,the uranium found on Earth contains 0.720%![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents