The wave function for an electron that is confined to x ≥ 0 nm is
ψ(x) = 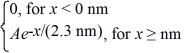
(a) What must be the value of A?
(b) What is the probability of finding the electron in the interval 1.15 nm ≤ x ≤ 1.84 nm?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q2: The square of the wave function of
Q4: If the accuracy in measuring the position
Q5: An electron inside a hydrogen atom is
Q6: A nonrelativistic proton is confined to a
Q8: A set of five possible wave functions
Q11: The wave function for a particle must
Q16: A small dust particle of mass 7.90
Q16: A nonrelativistic electron is confined to a
Q17: If the accuracy in measuring the velocity
Q17: A measurement of an electron's speed is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents