A cylinder contains 1.2 moles of ideal gas, initially at a temperature of 116°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 6.4 x 105 Pa on the gas. The gas is cooled until its temperature has decreased to 27°C For the gas 
and the ideal gas constant 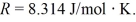
(a) Find the work done by (or on) the gas during this process. Is the work done by or on the gas?
(b) What is the change in the internal (thermal) energy of the gas during this process?
(c) How much heat is transferred to (or from) the gas during this process? Does this heat flow into or out of the gas?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q22: If we use 67 W of power
Q24: A 648-g empty iron kettle is put
Q27: In an isochoric process,the internal (thermal)energy of
Q29: It is necessary to determine the specific
Q32: The gas in a perfectly insulated system
Q39: In an isochoric process,the internal (thermal)energy of
Q45: The figure shows the pV diagram for
Q45: During an adiabatic process, 20 moles of
Q49: A cylinder contains 23 moles of an
Q51: A fixed amount of ideal gas goes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents