Heat is added to a pure substance in a closed container at a constant rate. The figure shows a graph of the temperature of the substance as a function of time. If Lf = latent heat of fusion and Lv = latent heat of vaporization, what is the value of the ratio Lv / Lf for this substance? 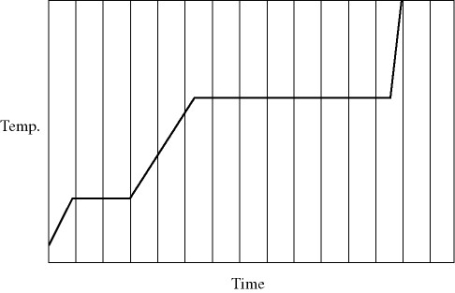
A) 5.0
B) 4.5
C) 7.2
D) 3.5
E) 1.5
Correct Answer:
Verified
Q30: A 406.0 kg copper bar is put
Q30: A block of ice at 0.000°C is
Q33: Two experimental runs are performed to determine
Q33: A 905-g meteor impacts the earth at
Q36: A 400-g piece of metal at 120.0°C
Q37: A person makes ice tea by adding
Q39: A person pours 330 g of water
Q40: Two experimental runs are performed to determine
Q56: A copper cylinder with a mass of
Q67: If you add 700 kJ of heat
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents