A vertical tube that is closed at the upper end and open at the lower end contains an air pocket. The open end of the tube is under the water of a lake, as shown in the figure. When the lower end of the tube is just under the surface of the lake, where the temperature is 37°C and the pressure is 1.0 × 105 Pa, the air pocket occupies a volume of 630 cm3. Suppose now that the lower end of the tube is at a depth of 86 m in the lake, where the temperature is 7.0°C. What is the volume of the air pocket under these conditions? The density of the water in the lake is 1000 kg/m3. 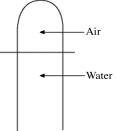
Correct Answer:
Verified
Q3: The number of molecules in one mole
Q8: If a certain sample of an ideal
Q8: (a)Internal human body temperature is often stated
Q11: A sealed 89-m3 tank is filled with
Q12: 2.0 L of a ideal nitrogen gas
Q15: A cold trap is set up to
Q17: A fixed amount of ideal gas is
Q19: Which contains more moles of material: 80
Q24: An ideal gas is at a pressure
Q38: A 3.2-L volume of neon gas (Ne)is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents