The figure shows a pV diagram for 0.0066 mol of gas that undergoes the process 1 → 2 → 3. What is the pressure p2. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 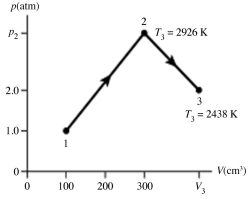
A) 5.3 atm
B) 5.3 × 105 atm
C) 16 atm
D) 1.6 × 106 atm
Correct Answer:
Verified
Q20: The figure shows a 50-kg frictionless cylindrical
Q21: The figure shows a pV diagram for
Q21: The interior of a refrigerator has a
Q25: What is the mass density of argon
Q26: The figure shows a pV diagram for
Q28: The figure shows a pV diagram for
Q31: A sealed container holds 0.020 moles of
Q35: How many moles of water (H2O)molecules are
Q38: A 3.2-L volume of neon gas (Ne)is
Q45: A 25-L container holds ideal hydrogen (H2)gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents