Phosphoenolpyruvate could undergo hydrolysis to form pyruvate and hydrogen phosphate.This reaction could be represented as shown below.(ΔG°' = -61.9 kJ/mol) 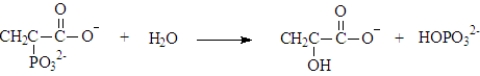 This reaction can be used to phosphorylate ADP.Write the net reaction when this reaction is coupled with ADP phosphorylation and calculate ΔG°' for the reaction.
This reaction can be used to phosphorylate ADP.Write the net reaction when this reaction is coupled with ADP phosphorylation and calculate ΔG°' for the reaction.
Correct Answer:
Verified
Q16: Exhibit 29-1
MATCH a term or structure from
Q17: Exhibit 29-1
MATCH a term or structure from
Q18: Exhibit 29-3
Use the data below to answer
Q19: Exhibit 29-3
Use the data below to answer
Q20: Exhibit 29-1
MATCH a term or structure from
Q22: Draw the structure of the product formed
Q23: Which of the following does not correctly
Q24: In coupled reactions,the net reaction has:
A)ΔG =
Q25: What type of high energy bonds are
Q26: During the synthesis of fatty acids,the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents