Explain why nonafluoro-2-methyl-2-propoxide is a much weaker base than tert-butoxide. 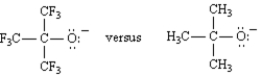
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: Draw: 2-phenyl-2-propanol
Q2: Exhibit 17-2
Refer to the data below to
Q3: Exhibit 17-3
To answer the following question(s), consider
Q4: Exhibit 17-4
To answer the following question(s),consider the
Q6: Exhibit 17-3
To answer the following question(s), consider
Q7: H2O2,−OH
Q8: Exhibit 17-4
To answer the following question(s),consider the
Q9: Exhibit 17-3
To answer the following question(s), consider
Q10: Draw: cis-4-tert-butylcyclohexanol
Q11: Name:
HOCH2CH2OH
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents