Exhibit 16-3
Consider the data below to answer the following question(s).
The −NH2 group is listed in our textbook as the strongest o,p-directing activator in electrophilic aromatic substitution reactions.However,when aniline is subjected to standard nitration conditions poor yields of m-nitroaniline result. 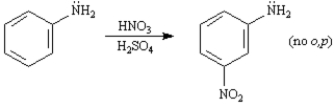
-Refer to Exhibit 16-3.Draw all the resonance forms of aniline showing the electron-donating effect of the −NH2 substituent.
Correct Answer:
Verified
Q1: Exhibit 16-2
Consider the Friedel-Crafts alkylation reaction below
Q2: Exhibit 16-1
MATCH a structure or term from
Q3: Exhibit 16-1
MATCH a structure or term from
Q4: Exhibit 16-1
MATCH a structure or term from
Q6: Aniline reacts with nitrous acid,HNO2,to yield a
Q7: Exhibit 16-2
Consider the Friedel-Crafts alkylation reaction below
Q8: Exhibit 16-6
To answer the following question(s),refer to
Q9: Exhibit 16-1
MATCH a structure or term from
Q10: Would you expect (nitromethyl)benzene to be more
Q11: Exhibit 16-2
Consider the Friedel-Crafts alkylation reaction below
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents