Consider the two lines shown on the energy diagram below. 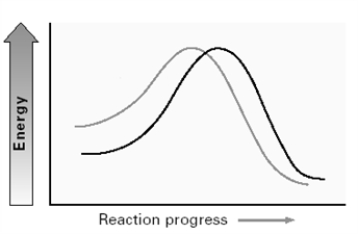 In an SN2 reaction,
In an SN2 reaction,
A) the upper left line could represent Cl- and the lower left line I-.
B) the upper left line could represent OH- and the lower left line CH3COO- .
C) the upper left line could represent H2O and the lower left line H2S.
D) the upper left line could represent (CH3) 2NH and the lower left line (CH3) 2N-.
Correct Answer:
Verified
Q19: Exhibit 11-4
Consider the pair of reactions below
Q20: Exhibit 11-8
Consider the reaction below to answer
Q21: For the elimination reaction: Q22: Draw the E2 mechanism of the reaction![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents