Exhibit 10-2
To answer the following question(s) consider the reaction below: 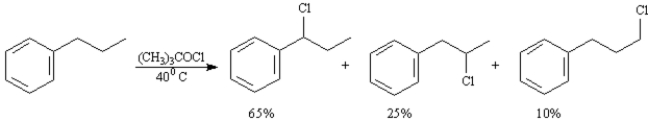
-A)When propylbenzene reacts with tert-butylhypochlorite three monochlorinated products are formed in the ratios indicated.Calculate a reactivity order for each type of hydrogen atom in propylbenzene.
B)The reaction of propylbenzene with tert-butylhypochlorite proceeds by a radical substitution pathway.Draw the structure of the radical intermediate leading to each product.
C)Based on your answers to the two questions above explain why (1-chloropropyl)benzene is the major product of this reaction.
Correct Answer:
Verified
Ar − CH2− = 65% ÷ 2 = 32.5% product ÷ 3...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q5: Exhibit 10-3
Consider the reaction below to answer
Q6: Exhibit 10-3
Consider the reaction below to answer
Q7: Exhibit 10-2
To answer the following question(s) consider
Q8: Draw the skeletal structure of the product
Q9: Rank the following compounds in order of
Q11: In the discussion on relative reactivity of
Q12: Predict the product of the following reaction.
Q13: Draw: (S)-2-bromobutane
Q14: Draw: 1,2-dichloro-1,1,2,2-tetrafluoroethane (Cryofluorane)
Q15: Draw: 3-iodopropene
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents