Exhibit 8-9
To answer the question(s) below consider the following information:
In an abandoned laboratory has been found a flammable liquid,A,in a bottle bearing only the label "Compound A: C7H12." Government agents have offered you a considerable sum to determine the structure of this compound.After verifying the molecular formula by elemental analysis,you find that Compound A reacts with 1 mol equiv of hydrogen;and,after treatment with acidic KMnO4,Compound A gives the dicarboxylic acid C (see below).Another bottle from the same laboratory is labeled "Compound B (isomer of A)." Compound B also reacts with 1 mol equiv of hydrogen,but yields cyclohexanone after treatment with acidic KMnO4.
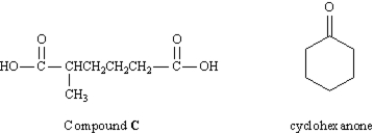
-Refer to Exhibit 8-9.What was the other product formed in the KMnO4 oxidation of B?
Correct Answer:
Verified
Q23: Which of the following is a difference
Q24: Draw the skeletal structure(s) and name the
Q25: Consider partial hydrogenation of the following substance.
Q26: Consider the polymeric structure below for amylose
Q27: Consider the following polymer. Q29: Draw the mechanism of the bromination of Q30: Consider the following intermediate. Q31: Draw the skeletal structure(s) and name the Q32: Which of the following will not undergo Q33: In the formation of an addition polymer![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents