Consider the following table. 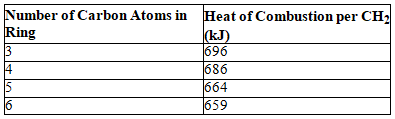
Based on the data in the table,which of the following compounds would have the largest strain energy?
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
Correct Answer:
Verified
Q26: Consider the following table. Q27: D-Pinitol is an interesting hexahydroxy cyclohexane,whose structure Q28: In methylcyclohexane: Q29: Consider the two methyl groups indicated with Q30: Which of the following would produce the Q32: What relationship exists between the following two Q33: The two structures show below represent: Q34: Exhibit 4-4 Q35: Exhibit 4-4 Q36: Exhibit 4-4![]()
A)all carbon atoms are sp3 hybridized.
B)ring-carbon
Label each pair of compounds below
Label each pair of compounds below
Label each pair of compounds below
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents