What is the orbital radius of the 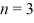 excited state in the Bohr model of the hydrogen atom? The ground-state radius of the hydrogen atom is 0.529 × 10-10 m.
excited state in the Bohr model of the hydrogen atom? The ground-state radius of the hydrogen atom is 0.529 × 10-10 m.
A) 0.477 nm
B) 0.159 nm
C) 0.382 nm
D) 0.549 nm
Correct Answer:
Verified
Q12: What is the frequency of the light
Q18: Light excites atomic hydrogen from its lowest
Q36: A hydrogen atom initially in the n
Q37: X-rays of energy 2.9 × 104 eV
Q39: The Bohr radius of the hydrogen atom
Q41: Light of wavelength 105 nm falls on
Q42: A gas of helium atoms (each of
Q43: A nonrelativistic electron has a kinetic energy
Q44: A nonrelativistic electron is accelerated from rest
Q45: A single slit is illuminated at normal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents