A hydrogen atom makes a downward transition from the 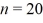 state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
A) 2.43 μm
B) 1.46 μm
C) 1.94 μm
D) 2.92 μm
Correct Answer:
Verified
Q16: Suppose that in a parallel universe,the proton
Q20: A laser emits light of wavelength 463
Q20: The energy of the ground state in
Q21: Light shines through atomic hydrogen gas.It is
Q22: Calculate the kinetic energy (in eV)of a
Q23: In a double slit experiment,a beam of
Q27: Electrons emerge from an electron gun with
Q28: What is the energy of a photon
Q29: A photon of wavelength 18.0 pm is
Q30: A photon of wavelength 29 pm is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents