A heat engine takes 2.0 moles of an ideal gas through the reversible cycle abca,on the pV diagram shown in the figure.The path bc is an isothermal process.The temperature at c is 820 K,and the volumes at a and c are 0.010 m3 and 0.16 m3,respectively.The molar heat capacity at constant volume,of the gas,is 37 J/mol ∙ K,and the ideal gas constant is R = 8.314 J/(mol ∙ K) .The thermal efficiency of the engine is closest to 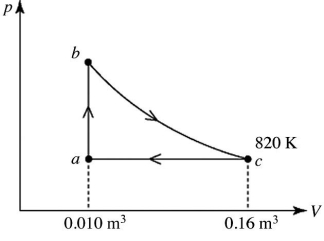
A) 0.26.
B) 0.026.
C) 0.33.
D) 0.40.
E) 0.53.
Correct Answer:
Verified
Q1: During each cycle of operation,a refrigerator absorbs
Q2: A real (non-Carnot)heat engine,operating between heat reservoirs
Q2: A certain engine extracts 1300 J of
Q3: A heat engine with an efficiency of
Q9: A Carnot engine operating between a reservoir
Q13: Is it possible to transfer heat from
Q18: A refrigerator has a coefficient of performance
Q23: A certain Carnot heat pump transfers energy
Q31: An ideal reversible refrigerator keeps its inside
Q36: An air conditioner with a coefficient of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents