A 610-g quantity of an ideal gas undergoes a reversible isothermal compression at a temperature of 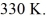 The compression reduces the volume of the gas from
The compression reduces the volume of the gas from 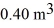 initially,to
initially,to 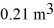 finally.The molecular mass of the gas is
finally.The molecular mass of the gas is 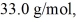 and the ideal gas constant is R = 8.314 J/(mol ∙ K) .The entropy change for the gas is closest to
and the ideal gas constant is R = 8.314 J/(mol ∙ K) .The entropy change for the gas is closest to
A) -99 J/K.
B) -81 J/K.
C) 99 J/K.
D) 81 J/K.
E) 0.00 J/K.
Correct Answer:
Verified
Q4: An engine manufacturer makes the claim that
Q20: A nuclear fission power plant has an
Q21: A Carnot engine is operated as a
Q21: A Carnot air conditioner operates between an
Q25: What is the change in entropy of
Q26: What is the maximum theoretical efficiency possible
Q35: The compressor in a certain Carnot refrigerator
Q37: A Carnot cycle engine operates between a
Q44: A system consists of two very large
Q45: A 2.00 kg piece of lead at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents