What is the average translational kinetic energy per molecule of an ideal gas at a temperature of 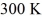 ? The Boltzmann constant is 1.38 × 10-23 J/K.
? The Boltzmann constant is 1.38 × 10-23 J/K.
A) 1.7 × 10-21 J
B) 8.3 × 10-21 J
C) 6.2 × 10-21 J
D) 2.1 × 10-21 J
E) 4.1 × 10-21 J
Correct Answer:
Verified
Q29: The root-mean-square speed (thermal speed)of the molecules
Q30: On a hot summer day,the temperature is
Q31: A 0.10- Q33: A cubic box with sides of 20.0 Q35: A sealed container holds 0.020 moles of Q39: What is the mean free path for Q42: Eleven molecules have speeds 16,17,18,...,26 m/s.Calculate the Q45: What is the mean free path of Q52: What is the root-mean-square value of the Q53: The root-mean-square speed (thermal speed)of the molecules![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents