How many grams of ice at -13°C must be added to 711 grams of water that is initially at a temperature of 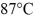 to produce water at a final temperature of
to produce water at a final temperature of 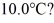 Assume that no heat is lost to the surroundings and that the container has negligible mass.The specific heat of liquid water is 4190 J/kg ∙ °C and of ice is 2050 J/kg · °C.For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg.The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Assume that no heat is lost to the surroundings and that the container has negligible mass.The specific heat of liquid water is 4190 J/kg ∙ °C and of ice is 2050 J/kg · °C.For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg.The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Correct Answer:
Verified
Q4: When a fixed amount of ideal gas
Q12: An ideal gas increases in temperature from
Q25: If 2.0 g of water at 0.00°C
Q26: What is the steady state rate of
Q28: Two metal rods,one silver and the other
Q33: Two experimental runs are performed to determine
Q36: A 400-g piece of metal at 120.0°C
Q39: A person pours 330 g of water
Q40: Two experimental runs are performed to determine
Q41: A solid concrete wall 4.0 m by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents