The figure (not to scale) shows a pV diagram for 1.8 g of helium gas (He) that undergoes the process 1 → 2 → 3.Find the value of V3.The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K,and the atomic weight of helium is 4.0 g/mol. 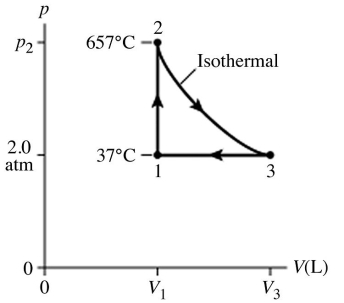
A) 17 L
B) 69 L
C) 34 L
D) 8.6 L
Correct Answer:
Verified
Q8: A steel container,equipped with a piston,contains 21
Q34: During an isothermal process,5.0 J of heat
Q44: The walls of an ice chest are
Q48: A cube at 100.0°C radiates heat at
Q53: A blacksmith is flattening a steel plate
Q54: A spherical object 25.0 cm in diameter
Q55: The figure shows a pV diagram for
Q56: Some properties of glass are listed here.
Q60: A heat conducting rod,1.40 m long,is made
Q62: The figure shows a pV diagram for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents