If a certain sample of an ideal gas has a temperature of 109°C and exerts a pressure of 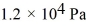 on the walls of its container,how many gas molecules are present in each cubic centimeter of volume? The ideal gas constant is 8.314 J/mol · K and Avogadro's number is
on the walls of its container,how many gas molecules are present in each cubic centimeter of volume? The ideal gas constant is 8.314 J/mol · K and Avogadro's number is
6.022 × 1023 molecules/mol.
Correct Answer:
Verified
Q9: A weather balloon contains 12.0 m3 of
Q24: An ideal gas is at a pressure
Q26: A sealed 89- Q27: 2.0 L of an ideal nitrogen gas Q28: The figure shows a 50-kg frictionless cylindrical Q30: The coefficient of volume expansion of olive Q31: A sealed container holds 0.020 moles of Q33: A vertical tube that is closed at Q34: 3.00 moles of an ideal gas at Q35: A certain metal has a coefficient of![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents