Which would be true about the following reaction? M + N 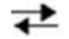 Y + Z
Y + Z
A) Adding a catalyst would alter the final concentrations of products and reactants at equilibrium.
B) Starting at chemical equilibrium, increasing the concentration of M will transiently increase the rate of formation of Y and Z.
C) Starting at chemical equilibrium, decreasing the concentration of M will increase the concentration of Y and Z.
D) Both the reaction is reversible and at chemical equilibrium and increasing the concentration of M will drive the reaction to the left are correct.
E) Because the reactants and products are different molecules, this is not a reversible reaction.
Correct Answer:
Verified
Q22: Which is the best definition of the
Q22: A solution containing proteins of a particular type is
Q23: What is "allosteric modulation"?
A) regulation of physiological
Q24: A certain protein receptor is capable of
Q25: What is the term for the segments
Q26: How does the synthesis of proteins that
Q28: Which is a function of transfer RNA
Q32: Which is NOT a function of the
Q39: In skeletal muscle,when calcium binds to the
Q52: The term "metabolism"
A)is synonymous with the term
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents