Consider the reaction: H2CO3 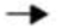 CO2 + H2O + 4 kcal/mol. Which of the following is TRUE?
CO2 + H2O + 4 kcal/mol. Which of the following is TRUE?
A) The reaction is anabolic and the energy content of the reactant is greater than that of the products.
B) The reaction is catabolic and the energy content of the reactant is greater than that of the products.
C) The reaction is anabolic and the energy content of the products is greater than that of the reactant.
D) The reaction is catabolic and the energy content of the products is greater than that of the reactant.
E) The reaction is catabolic and the energy content of the products are equal to that of the reactant.
Correct Answer:
Verified
Q41: What is the function of a catalyst
Q45: Enzymes
A)are catalysts in chemical reactions.
B)can be carbohydrate
Q47: Which of the following is NOT a
Q49: Which of the following is true concerning
Q50: Which is NOT true about fatty acid
Q51: Which of the following metabolic pathways can
Q52: Most energy in the body is stored
Q53: Which is a series of reactions by
Q57: Which is NOT true about cofactors involved
Q65: What are the products of glycolysis under
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents