Phosphoric acid (H3PO4)has three dissociable protons,with the pKa's shown below.Which form of phosphoric acid predominates in a solution at pH 4? Explain your answer.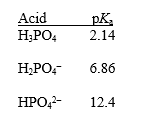
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q33: You want to maintain pH = 7.0
Q36: H+ + HCO3-
Q64: What is the pH of a solution
Q65: Explain the fact that ethanol (CH3CH2OH) is
Q70: Explain the fact that triethylammonium chloride ((CH3CH2)3N-HCl)
Q74: Explain with an appropriate diagram why amphipathic
Q80: You have just made a solution by
Q82: In proteins, the amino acid histidine (His)
Q86: A weak acid HA, has a pKa
Q87: If ice were denser than water, how
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents