During transfer of two electrons through the mitochondrial respiratory chain, the overall reaction is:
NADH + 1/2 O2 + H+ NAD+ + H2O
For this reaction, the difference in reduction potentials for the two half-reactions ( E'°) is +1.14 V. Show how you would calculate the standard free-energy change, G'°, for the reaction. (The Faraday constant, 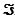 , is 96.48 kJ/V · mol.)
, is 96.48 kJ/V · mol.)
Correct Answer:
Answered by Quizplus AI
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q82: The free energy of hydrolysis of
Q83: Explain what is meant by the statement:
Q84: glucose 6-phosphate
Q85: The first law of thermodynamics states that
Q86: The standard free energy change (
Q88: What is the difference between
Q89: Explain in quantitative terms the circumstances
Q90: Consider the reaction: A + B
Q91: Why is the actual free energy
Q92: Given
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents