(a)On the reaction coordinate diagram shown below,label the transition state and the overall free-energy change ( G)for the uncatalyzed reaction A B.
(b)Is this an exergonic or endergonic reaction?
(c)Draw a second curve showing the energetics of the reaction if it were enzyme-catalyzed. 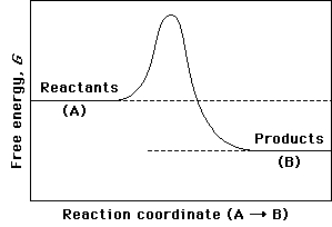
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q46: (a)What is optical activity?
(b)How did Louis Pasteur
Q82: Discuss how a mutation in DNA could
Q85: The free-energy change for the formation of
Q86: Describe the relationship between a living organism
Q92: Hereditary transmission of genetic information can be
Q93: A chemist working in a pharmaceutical lab
Q94: Proteins are constantly being synthesized in a
Q101: Describe Stanley Miller's experiment (1953) and its
Q106: What is meant by endosymbiotic association? How
Q108: Describe how the rise of O2-producing bacteria
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents