Which of the following best describes the bond length of the central sigma bond in 1,3-butadiene shown below? 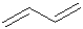
A) It is longer than an average C-C sigma bond.
B) It is shorter than an average C-C sigma bond.
C) It is the same length as an average C-C sigma bond.
D) There is not enough information to determine bond length.
Correct Answer:
Verified
Q8: Which of the following best represents the
Q9: How many nitrogen lone pairs are involved
Q10: How many atoms are part of the
Q11: Which of the following compounds is considered
Q12: Which of the following is NOT a
Q14: Which of the following best describes the
Q15: Why does an increase in conjugation lead
Q16: Which molecule would be expected to have
Q17: Compared to ethene,butadiene would be expected to
Q18: Which of the following ions is considered
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents