Both pyridine and pyrrole shown below have a lone pair of electrons on their nitrogen capable of acting as a base.Which compound would you expect to be the stronger base.Why? 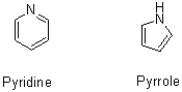
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q59: Anti-aromatic compounds are also considered non-aromatic.
Q60: Assuming planarity,the following molecule is considered non-aromatic.
Q61: Benzene has a _ heat of hydrogenation
Q62: A molecule that is anti-aromatic has _
Q63: Aromatic double bonds tend to be _
Q65: The hybridization of a nitrogen atom in
Q66: LUMO is an acronym for the _
Q67: _ circles are a method of mapping
Q68: Indicate which atoms are part of the
Q69: The following molecule shown below is Lipitor,a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents